Institute of Industrial Science, The University of Tokyo
Professor
Link to Affiliation: https://www.iis.u-tokyo.ac.jp/ja/research/staff/masaru-ogura/
Research and development of the emission reduction and resourcing of nitrogen oxides (NOx) in exhaust gas
Our group is focusing on and developing a technology to recover nitrogen oxides (NOx) contained in flue gas and convert them into resources that we call “NOx to Ammonia: NTA,” which are not typically recovered in a rational manner due to their low concentration.
- Ultra-selective adsorption materials for NO using metal species–derived electrostatic fields in hydrophilic/hydrophobic spaces (IIS, University of Tokyo)
- Precise nano-space design using porous oxide materials and the provision of NOx storage field for NTA (National Institute of Advanced Industrial Science and Technology (AIST))
- Development of a highly active catalyst for NTA at low temperatures using an unburned reducing agent or excess hydrogen under anoxic conditions (National Institute of Advanced Industrial Science and Technology (AIST))
- Development of catalyst/catalysis technologies for NTA using unburned hydrocarbons under oxygen-rich conditions (Waseda University)
- Research and development (R&D) of NH3 adsorption and resource recovery after the NTA process (National Institute of Advanced Industrial Science and Technology (AIST))
- Evaluation of single or integrated NTA processes (Tokyo Institute of Technology)

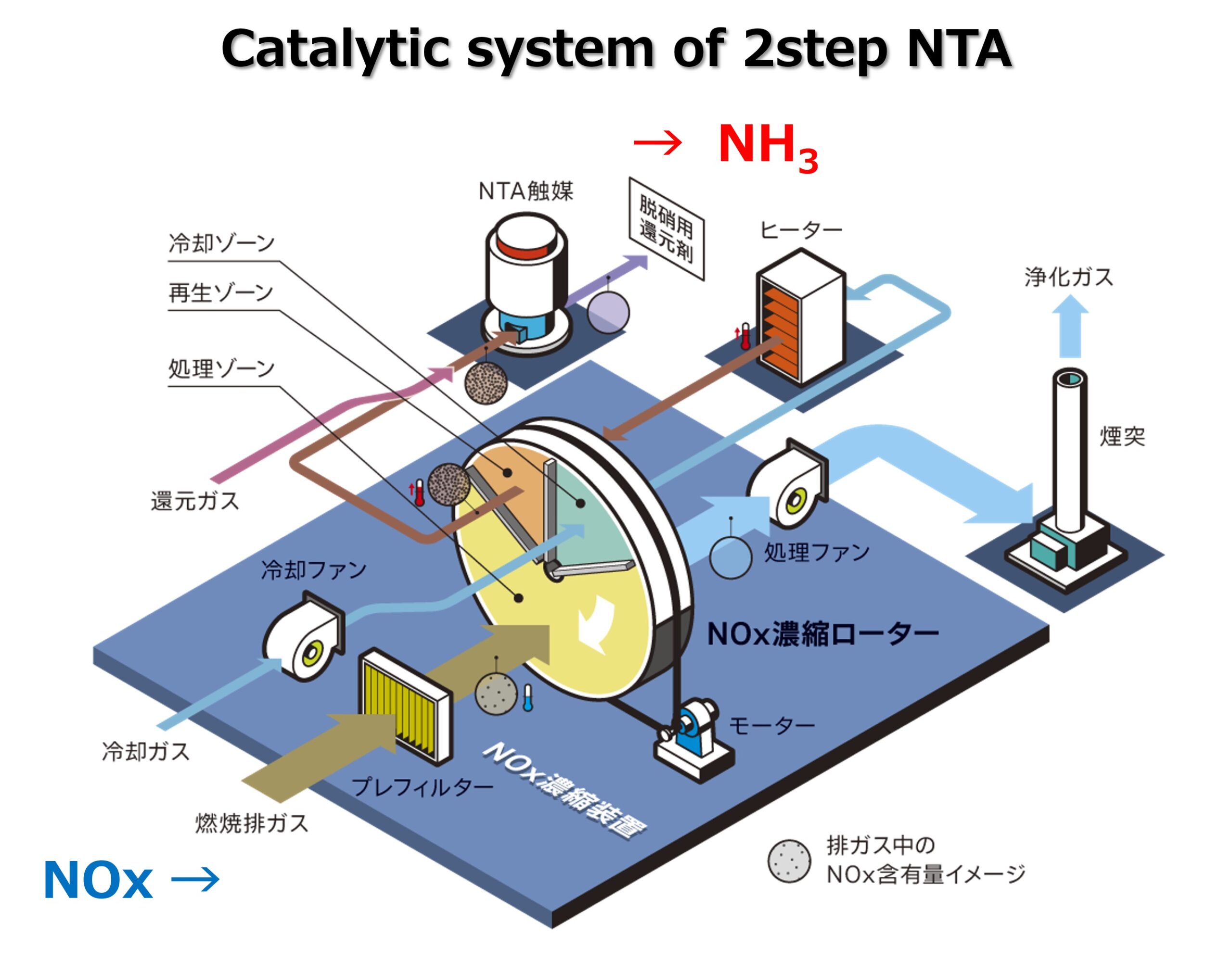
Design of a seamless two-stage NTA catalyst system
Our team has already succeeded in developing the following two systems of NTA catalysts for exhaust gas from internal combustion engines and waste treatment.
- An ultra-selective catalyst that promotes the NTA reaction in a single pass, even in the presence of relatively high contaminant concentrations.
- A non-selective catalyst with almost 100% NH3 yield in the absence of oxygen.
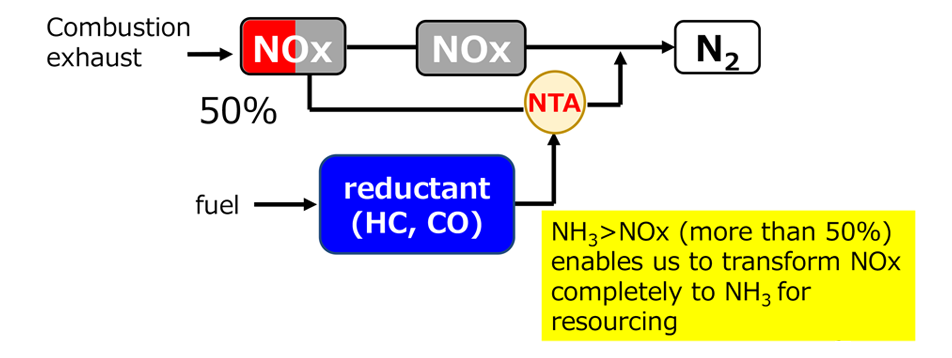
In this study, we pursue the systematization of Process 2 in particular. The catalytic reaction, which is difficult to achieve in Process 1, is divided into two reaction steps at different temperatures.- NO enrichment using a material that selectively and irreversibly adsorbs only NO in exhaust gas in the presence of relatively high concentrations of contaminants.
- The NTA reaction wherein highly concentrated NO is desorbed by heating and propelled in the absence of oxygen using a reductant, such as hydrogen.
We are designing a catalytic system that seamlessly promotes these two-stage surface reactions by dividing the reactions/functions into two stages: concentration and surface reactions.
Development of catalyst/catalysis technologies for NTA using unburned hydrocarbons under oxygen-rich conditions
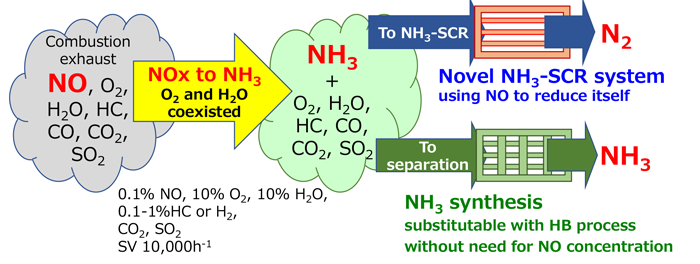
The goal is to construct a catalytic reaction system that can selectively reduce nitrogen oxides (mainly NO) to NH3, even in the presence of large amounts of oxygen, water vapor, carbon dioxide, sulfur dioxide, and other foreign substances.
When the NH3 yield reaches 50%, NH3-SCR operation using the generated NH3 as the reductant for NO becomes feasible. Meanwhile, if the NH3 yield can be increased to >50%, a new NH3 synthesis approach can be realized using a separation and concentration process in the subsequent stage. In either case, the process does not require raw gas purification and the high-pressure reactions of conventional Haber–Bosch NH3 synthesis, improving its energy efficiency.
In addition, this method can reduce the release of harmful nitrogen compounds into air.